What is Zinc Metal?
Zinc is commonly known by the German term zink. It has been utilized for a very long time before anyone even considered elements, and it has been known since the prehistoric era. A blue-white metal with average strength, hardness, and ductility is zinc (Zn). One of the least frequent elements, zinc is primarily created by electrolyzing aqueous zinc sulfate. Galvanized nails are made using about a third of all metallic zinc. It serves to coat the metal and shield it from corrosion due to its low melting point and capacity to establish bonds with iron or steel. Batteries made on dry cells can also be made using metallic zinc.
Origin and history of Zinc Metal:
The history of metallic zinc is substantially later than that of the other major metals. By heating their oxide ores with charcoal (carbon), a process known as reduction, in shaft furnaces, which were invented quite early in history, copper, lead, tin, and the iron can be produced as molten metals. However, until temperatures rise far above the metal’s comparatively low boiling point (907 °C), zinc oxide cannot be reduced by carbon. Zinc could not be produced in the furnaces designed to smelt the other metals. Lead blast furnace flues can include trace amounts of metallic zinc.
The Greeks may have known about zinc and named it “pseudargyras”, or “fake silver,” but they did not have a way to produce it in large quantities.
Brass, an alloy of zinc and copper, was manufactured in large quantities by the Romans by burning a combination of zinc oxide and charcoal covered with chunks of metallic copper. In the bottom of the crucible, the zinc oxide was reduced. Brass was created when zinc vapor was condensed and dissolved with copper. The temperature was increased after the procedure to melt the brass for casting into ingots. Zinc was solely used by the Romans to make brass.
The understanding that zinc must first be produced as a vapor and then condensed seems to have originally emerged in India in the 13th or 14th century. By the 16th century, China’s metallurgists had mastered the large-scale manufacturing of zinc. Under William Champion’s direction, this idea was first utilized in the West in England in 1743. Improvements were made to the furnace at the end of the 18th century in Belgium and Poland, but the procedure remained unaltered until an electrolytic process was invented in 1917. The furnace was improved at the end of the 18th century in Belgium and Poland, and the procedure remained constant until an electrolytic method was created in 1917. In the United States, a continuous retort method was created at the end of the 1920s, and in the 1930s, an electrothermic process for continuous zinc production was developed. The quick quenching of the gases is a basic principle of the zinc-lead blast furnace, a development of the 1960s. Zinc processing covers the zinc-producing procedures in great depth.
Properties of Zinc Metal:
The two types of properties of Zinc Metal can be classified as:
Physical Properties:
Appearance: Pure zinc is a bluish-silver, ductile metal with a low melting and boiling point. The majority of zinc used today is ZnS, which is derived from zinc blend ore after being roasted to burn off the sulfur. A frequent laboratory activity, electrolysis of aqueous zinc sulfate can also be used to extract zinc.
Strength: The tensile strength of zinc is less than half that of mild carbon steel, making it a weak metal. Despite being able to be die cast from affordable mechanical parts of zinc, it is typically not used in load-bearing applications.
Toughness: Compared to other die-casting alloys, zinc alloys often have higher impact strength than pure zinc, which has low toughness and is typically brittle.
Ductility: Zinc becomes ductile and pliable between 212 and 302 degrees Fahrenheit, but at higher temperatures, it reverts to a brittle form. Zinc alloys significantly outperform pure zinc in this regard, enabling the adoption of more intricate production techniques.
Conductivity: Zinc has a reasonable conductivity for a metal. However, its potent electrochemical characteristics make it useful in galvanizing and alkaline batteries. Zinc is used extensively in the manufacture of alloys. Brass, one of the most well-known zinc alloys, comprises 55–95% copper. Additionally, zinc is used in the production of solder, which has a relatively low melting point. Pipes and other metals, as well as electrical components, are all joined together with solder.
Chemical Properties: Zinc is used as a coating for inferior metals like iron since it has moderate resistance to corrosion (“galvanizing”). Zinc is easily castable or moldable. Zinc has a variety of distinctive qualities. For instance, its vapor produces zinc oxide when it burns in the air with a green flame. Common zinc compounds include zinc oxide, used in paints, cosmetics, plastics, and other products. With mild acids, metallic zinc reacts relatively slowly. Zinc and sulfur have strong chemical bonds. The two powders react violently to create zinc sulfide when heated. Fluorescent light bulbs and television screens are both made of zinc sulfide.
Halogens and zinc also react. However, the reactivity with zinc reduces as the electronegativity of the halogen group increases. Fluorine, the most electronegative halogen, severely reacts with zinc while iodine, the least electronegative halogen, just produces a small amount of heat. It’s interesting to note that contaminants like lead, cadmium, and iron have a significant impact on the characteristics of zinc. Furthermore, zinc is frequently employed as a reducing agent in chemical processes, where it produces complex ions with cyanide and ammonia ions.
Uses of Zinc Metal:
Zinc has many different uses across many industries due to its low cost, high strength, and low reactivity with other metals. It is used in automobile manufacturing for parts like brake lines, fuel tanks, and catalytic converters; it is also used in construction for roofing materials; and it is used in electronics for circuit boards and as protective coating for electrical wires. Additionally, zinc can be alloyed with other metals such as copper or aluminum to make them stronger or more resistant to corrosion or rusting.
Because of its versatility and low cost, zinc metal can be found in virtually everything from coins to cookware to electronics components. Its ability to form strong alloys with other metals makes it especially useful in the production of automobiles and airplanes where strength must be balanced with durability. Furthermore, due to its anti-corrosive properties it is often used as a protective coating on steel structures such as bridges or buildings that are exposed to harsh weather conditions or saltwater environments. Finally, zinc oxide powder can be applied topically as an antiseptic cream or ointment for minor skin irritations such as cuts or burns due to its natural anti-inflammatory properties.
The metal is utilized in manufacturing processes to make things like zinc oxide and roofing materials.
The metallic element is employed in a variety of products, including sunscreen, solar cells, and nuclear reactors.
This metal supports the body’s ability to keep its balance of enzymes.
For oil-based paints, it serves as a white pigment.
The addition of zinc oxide to the rubber used to create car tires is another significant application.
Zinc oxide can tolerate high temperatures and keeps tires from disintegrating at high temperatures.
Freshly cast zinc has a bluish silver surface that gradually turns into a protective oxide film in the air. High purity zinc (99.99 percent) is ductile, while the so-called prime western grade (99.8 percent pure) is brittle at room temperature but may be rolled into flexible sheets at temperatures above 100 °C (212 °F). Zinc crystallizes as a densely packed hexagonal structure.
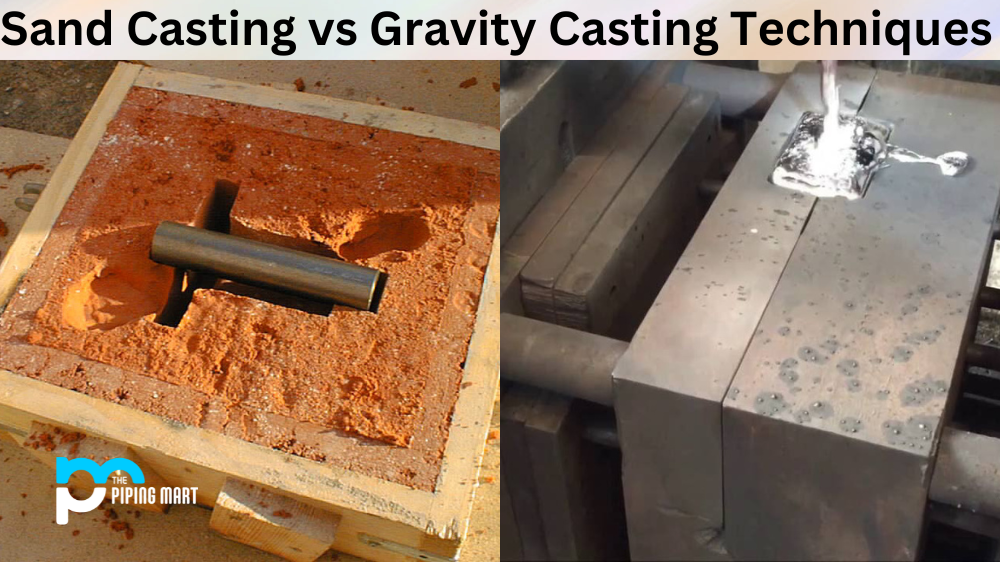
In the field of die casting, zinc is frequently employed. After iron, aluminum, and copper, zinc is currently the fourth most extensively consumed metal worldwide. It is well related to other metals and has potent anti-corrosive qualities.
Galvanizing, which involves coating iron or steel with thin layers of zinc to prevent rusting, uses half of the zinc produced.
The next most popular application for zinc is in alloys, where it is combined with copper (to make brass) and other metals to create components for household appliances, automotive parts, and electrical systems. The creation of zinc oxide, the most important zinc chemical by volume of production, which is used as a protective skin ointment in the rubber industry, is the third large usage of zinc.
Additionally crucial to wellness is zinc. It is essential for the healthy development and growth of people, animals, and plants. The amount of zinc needed for the immune system and body’s enzymes to work properly is found in the adult human body, which includes between 2 and 3 grams of zinc.
Common Alloys of zinc metal:
Brass, which contains 3-45% Zn by weight, is used to make valves, hardware, and musical instruments.
Nickel silver, which contains 20% zinc by weight, is utilized in jewelry, cutlery, model train tracks, and musical instruments because of its brilliant silver look.
Alloys for zinc die casting typically incorporate tiny amounts (less than a few percentage points) of Pb, Sn, Cu, Al, and Mg to enhance the mechanical qualities and die casting characteristics. These alloys typically comprise >78% Zn by weight. It can be used to create tiny, delicate shapes that are suited for machine moving components. The least expensive of these alloys is known as pot metal, and it can be used as a less expensive alternative to steel.
Different Compounds of Zinc:
The +2 oxidation state of zinc is virtually always present in chemical compounds. There have been a few zinc compounds recorded that is in the +1 state, but none that are in the +3 state or higher.
One of the most significant zinc compounds is zinc oxide or ZnO. By burning zinc vapor in the air, it can be created in a state of high purity and a range of crystal shapes and sizes. Zinc oxide is commonly used as a heat sink in rubber due to its high heat conductivity and capacity. The lattice (i.e., the ordered structure created by the ions) in the zinc oxide crystal is an open one, with the zinc and oxygen ions taking up only 44% of the space.
By applying particular treatments, such as inserting foreign atoms or zinc atoms into the lattice’s vacancies, defects can be produced in the structure. Different electrical, photoelectrical, and catalytic capabilities are produced by treating zinc oxide crystals in this manner. As a result, phosphors for television tubes and fluorescent lamps are made using zinc oxide as a semiconductor. Because of how many compounds react to it, it can be used as a catalyst in processes like making synthetic rubber and methanol. Additionally, it is utilized in printing inks, cosmetics, paints, and polymers. Zinc oxide is used in several photocopying processes because it may have its electrical conductivity multiplied by several under the effect of light.
An intermediate component in the electrolytic process used to produce zinc from its ores is zinc sulfate or ZnSO4. It serves as a weed killer, a component in the production of viscose rayon, and a mordant in the dyeing process. ZnCl2, or zinc chloride, can be created directly or by evaporating the aqueous solution that results from a variety of processes. It is used as a drying agent and a flux because it is very deliquescent (water-absorbing). It is employed as a wood preservative in aqueous solutions. Sphalerite is a naturally occurring mineral that contains zinc sulfide, or ZnS. ZnS can be produced by reacting zinc salt solutions with hydrogen sulfide. Long employed as a white pigment, titanium dioxide has increasingly taken its place. When small amounts of copper, manganese, silver, or arsenic are added, zinc sulfide develops luminescent qualities. As a result, it has been utilized in fluorescent lighting, X-ray screens, and luminous dials for clocks and watches.
Processing of Zinc:
zinc processing, also known as zinc extraction from its ores and zinc metal or chemical compound preparation for application in a variety of goods.
Zinc (Zn), a metallic element with a hexagonal structure, can be handled and produced ductile at temperatures that are only marginally higher than ambient. Due to the development of an oxide deposit on its surface, it is greyish-white in solid form, but when cast or cut freshly, it appears bright and silvery. Due to two of its exceptional qualities namely, its high corrosion resistance and its ability to offer sacrificial protection by corroding in place of iron when in contact with iron galvanizing is its most significant use.
Unalloyed zinc has poor technical qualities because of its low melting point of 420 °C (788 °F), but it is widely utilized when alloyed. Brass alloys are created by adding up to 45% zinc to copper, while pressure die-casting and gravity-casting alloys with economic importance are created by combining zinc with aluminum. Zinc is used to construct the cans for dry-cell batteries, which are made of sheets. By alloying zinc with tiny amounts of copper and titanium, an increased strength sheet is created, which is used for many structures’ roofing and cladding.
Zinc compounds, especially zinc oxide, have numerous commercial and medicinal applications.
Even though inc ores are found all over the world, more than 40% of the production comes from North America and Australia. The most prevalent zinc-containing minerals are marmatite [(ZnFe)S], a ferrous form of zinc blende known as sphalerite (ZnS), and calamine or smithsonite, a zinc carbonate (ZnCO3).
Deposits of zinc have complicated geology. The majority of the time, aqueous solutions have been driven through porous strata at extremely high temperatures and pressures to dissolve minerals like zinc, lead, and others, which then precipitated as sulfides. Mining ore typically contains between 3 and 10% zinc. The lead sulfide mineral galena and trace amounts of cadmium sulfide are found in almost all ores. There is frequently chalcopyrite and copper-iron sulfide. Calcite, dolomite, and quartz are the three most prevalent gangue components.
Mining and Concentration:
Zinc ores are extracted using a variety of mining processes, including open-pit mining (mostly for oxidized ore bodies, which are found closer to the Earth’s surface) and conventional underground methods (used for the more deeply located sulfide ores). Cut-and-fill stopping, in which tunnels are dug to a moderate depth and branch away from the mine entrances, is the most popular underground ore extraction technique.
To get a concentrate appropriate for treatment, beneficiation is required due to the limited fraction of zinc sulfide minerals present in the ore. The sulfide mineral is separated from the impure elements, or gangue, by flotation separation, which is the most popular technique for achieving this concentration.
In this, The ore is first crushed to a diameter of roughly 1.9 centimeters (0.75 inches), mixed with water, and processed to a thickness of fewer than 0.1 millimeters in a ball mill. Water and the finely ground particles combine to form a slurry that travels from the mill to flotation cells or tanks where beaters stir the slurry in the presence of particular chemical reagents that suspend air bubbles in it. While the gangue is wetted by the chemical activity and sinks in the cell, the mineral particles adhere to the bubbles and float to the surface, generating an oily froth that is continuously skimmed. It is possible to focus on the separation of each mineral that makes up complex lead and zinc sulfides by using the right foaming agents.
Extraction and Refining:
Sintering and roasting: Both electrolysis and smelting, the two main extraction processes for the production of zinc, call for the elimination of sulfur in a highly exothermic oxidation reaction beforehand.
In fluidized-bed roasters, where finely divided and heated concentrate particles are suspended in a rising stream of air, concentrates are roasted for the electrolytic manufacture of zinc. A sulfuric acid plant receives a high-strength (10%) sulfur dioxide gas after the sulfur content is lowered to less than 0.5 percent. The method produces calcine in the form of tiny particles that are easily dissolved into solutions for further processing, and it is thermally efficient.
If the concentrate quality declines and in particular if the lead content exceeds 3%, the method previously described becomes challenging to operate. The zinc-lead blast furnace uses a sintering process to furnish its oxidized feed because of this and the requirement for a robust, lumpy feed. The crushed returning sinter is combined with fine concentrates to create a substance that contains about 6.5 percent sulfur. The sintered cake exiting the machine is broken into manageable lump sizes after being fed onto a moving grate and burned in an air updraft. The sinter is the perfect feed for the blast furnace due to its strength and hardness. A sulfuric acid plant receives a gas that contains 7.5% sulfur dioxide.
Electrolysis: The fundamental processes in this procedure are (1) creating a zinc sulfate solution by leaching zinc oxide calcines (made by roasting sulfide concentrates) in diluted sulfuric acid, (2) purifying the resultant solution, and (3) electrolyzing the purified solution.
Theoretically, electrolysis should lead to the creation of hydrogen at the cathode rather than the deposition of zinc since the voltage needed to deposit zinc from a zinc sulfate solution onto a cathode is roughly double that needed to break down water. However, when a zinc cathode is employed, overvoltage prevents hydrogen from being produced, and as a result, zinc is deposited.
The quality of the zinc sulfate electrolyte plays a critical role in determining the hydrogen overvoltage; the presence of some contaminants, even at extremely low concentrations, can significantly reduce the overvoltage and obstruct zinc deposition. As a result, two phases of extremely thorough electrolyte purification are required to complete the process. Iron is first removed as a solid residue in the form of either goethite or hematite oxides, or jarosite, a basic ferric sulfate. After that, the solution is cemented with zinc dust to eliminate any remaining metallic impurities, such as copper, nickel, cadmium, cobalt, and germanium. Lead-lined concrete cells with cathodes made of aluminum sheet and anodes of lead with 0.5–1.0 percent silver are used for electrolysis. Every 24 to 48 hours, the zinc deposits are removed from the cathodes and remelted in an induction furnace before being formed into ingots.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.